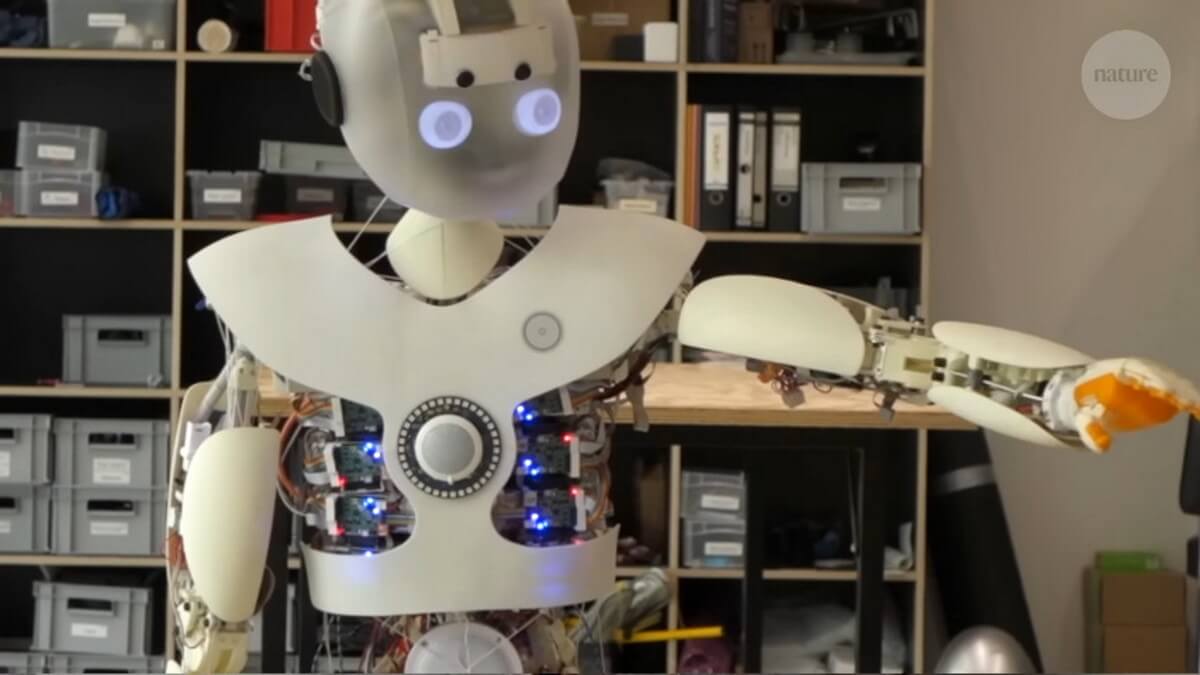
Human cells grown in a lab are the next medical frontier researchers are trying to get to. Now, a new approach is seeing human cells being grown on robotic skeletons.
The cells, which could one day be used for tissue grafts, need a certain type of stimulation to have the same stretching and twisting as they would experience in a real human body.
In the research titled “Humanoid robots to mechanically stress human cells grown in soft bioreactors,” scientists explain the challenges involved in creating human tissue and why torn tendons and other similar injuries are so difficult to heal.
“For more than 20 years, robotic bioreactor systems have facilitated the growth of tissue-engineered constructs using mechanical stimulation. However, we are still unable to produce functional grafts that can translate into clinical use,” the researchers explain.
To overcome this challenge, the scientists designed a flexible bioreactor chamber where they grow fibroblast cells. That chamber is attached to a musculoskeletal (MSK) humanoid robot shoulder joint.
Because human tissue and cells undergo incredible amounts of mechanical stresses like tension and compression, and the lack of those stresses can lead to their capability and size being reduced, lab-grown tendons have to undergo a similar treatment in order to be a viable candidate for implanting in humans.
Researchers chose a robotic shoulder for their project because torn rotator cuff tendons are the most common cause of shoulder pain in adults. According to them, over 25% of the population over 60 is affected by this, but the surgery required to repair those tendons has a failure rate of around 40%.
During the experiment in which they grew human cells on the robot, the researchers noted several differences from tissue usually grown in a static environment. However, the research is in its infancy and the researchers are not sure if those changes are in fact positive.
“We do get differences out of the loading regime [the movement of the bioreactor in the robot shoulder joint] but whether those differences mean better cells? We don’t know yet,” the lead researcher on the project, Pierre-Alexis Mouthuy of the University of Oxford’s Botnar Institute of Musculoskeletal Sciences, told The Verge.
Follow TechTheLead on Google News to get the news first.