Personal ECG devices have only been able to detect atrial fibrillation but that doesn’t cover all the other conditions that might be just as, or even more dangerous.
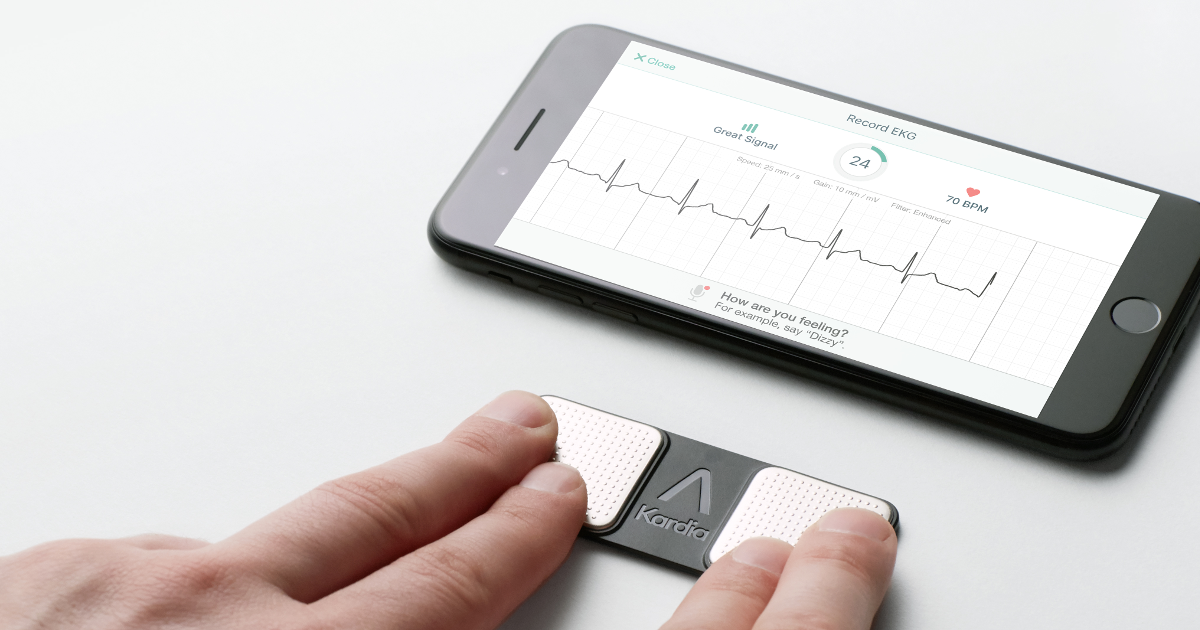
Thankfully, the KardiaMobile from AliveCor has been cleared by the FDA and it is the very first FDA-approved personal ECG device that can detect bradycardia (when the heart takes a dip down to 40-50BPM) and tachycardia (when instead, it jumps to 100-140BPM). These sort of conditions are sometimes indicators of heart disease and other issues.
“No other consumer ECG device in the world, can tell you more about your heart than KardiaMobile,” AliveCor chief Executive Officer Ira Bahr said “Until today, patients have been frustrated when devices label their ECG reading as ‘unclassified’ or ‘inconclusive.’ Starting today, KardiaMobile is the first personal ECG device that can begin to materially reduce the number of those determinations. Critically, KardiaMobile is also the only personal ECG that can detect Atrial Fibrillation at heart rates above 120, and heart rates below 40.”
AliveCor also insists that the device will only work to bring more information to the table when you visit your doctor, not completely replace them. Of course, the KardiaMobile is not as complex as the ECGs you can find in a hospital but it should work to provide a better picture of the user’s overall heart health.
Follow TechTheLead on Google News to get the news first.