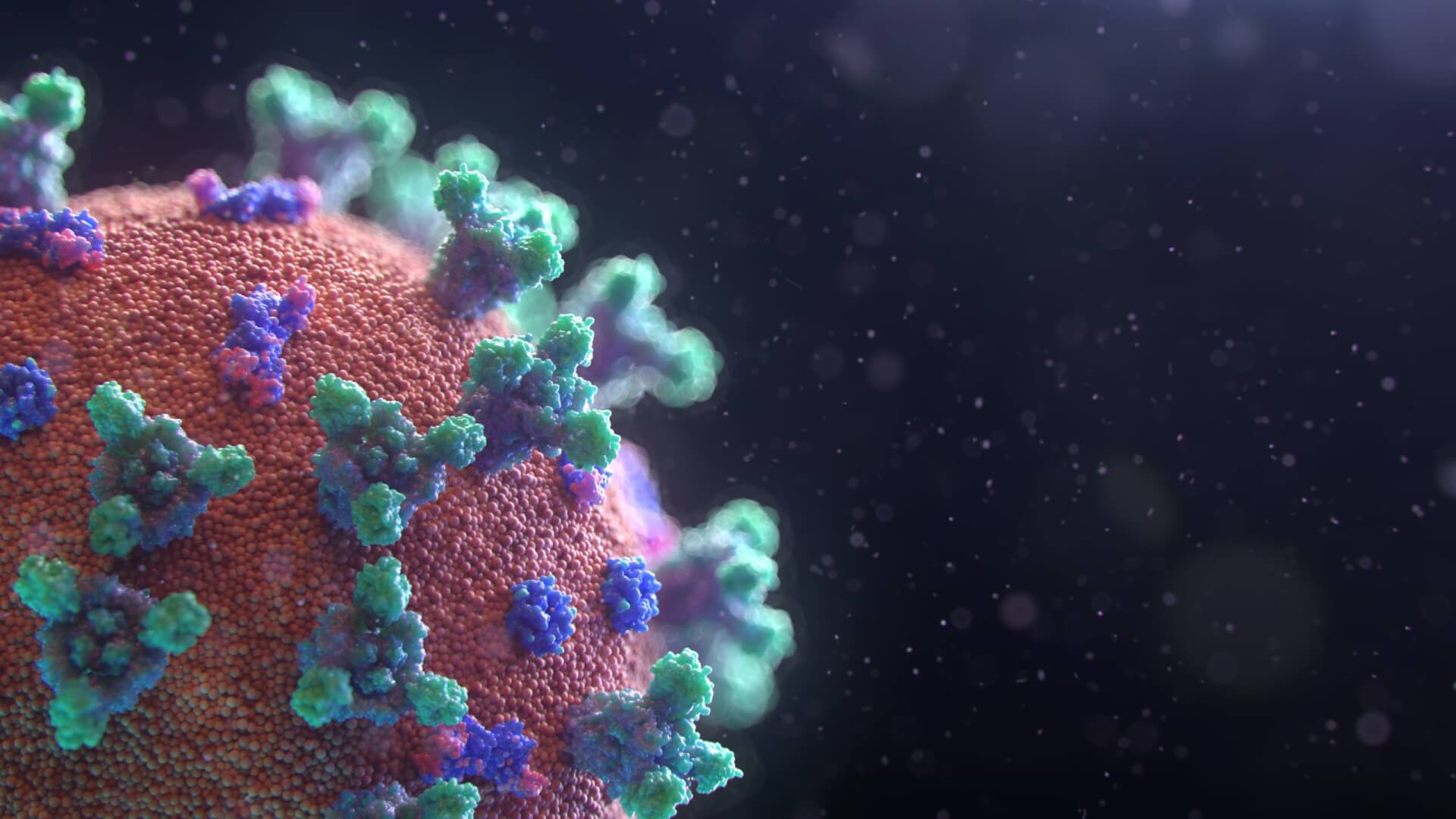
Developed by Advarra and Cambridge, a new mRNA vaccine boosts the immunity of trail patients infected with SARS-CoV-2. Emerging late 2019, the coronavirus, prompted a global rush to find a cure for the notorious acute respiratory syndrome that claimed the lives of countless people. With recent efforts to develop a way to counteract the virus, the mRNA-1273 proved to encode the stabilized prefusion SARS-CoV-2 spike protein, with this phase consisting of dose-escalation, open-label trial that includes 45 healthy adults, 18 to 55 years of age, resulting in better antibody responses with increasing doses.
The first trial was 29 days, and by the last day, researchers saw an increased antibody response. After the second vaccination, antibodies increased even more, and serum-neutralizing activity was detected by using two methods in all trial participants, to ensure an accurate result for the test.
To note, adverse events that occurred in more than half the participants included fatigue, chills, headache, myalgia, and pain at the injection site. It seems that systemic adverse events were more common after the second vaccination, particularly with the highest dose, and three participants of a particular dose group reported one or more severe adverse events.
The trial was reviewed and approved by the Advarra institutional review board and was supervised by an independent safety monitoring committee. All participants of the tests provided written consent before enrollment, and the trial was conducted under an Investigational New Drug application submitted to the Food and Drug Administration.
The vaccine was co-developed by researchers at the National Institute of Allergy and Infectious Diseases, the sponsor of the effort, and at Moderna, Cambridge, MA. The mRNA-1273 possible vaccine, if it hits the market, will be manufactured by Moderna, and will encode the S-2P antigen, consisting of the SARS-CoV-2 glycoprotein with a transmembrane anchor and an intact S1–S2 cleavage site. S-2P is stabilized in a prefusion conformation by two sequential proline exchanges at amino acid positions 986 and 987, at the top of the central helix in the S2 subunit. Normal saline was used as a diluent to prepare the doses administered.
Follow TechTheLead on Google News to get the news first.