[adrotate group=“15″]
A team of scientists from the University at Buffalo has developed a new 3D bioprinting technique that is up to 50 times faster than the second-best methods available. The researchers have thus managed to reduce the time required to create a cell-laden hydrogel structure to just 19 minutes from the average 6 hours.
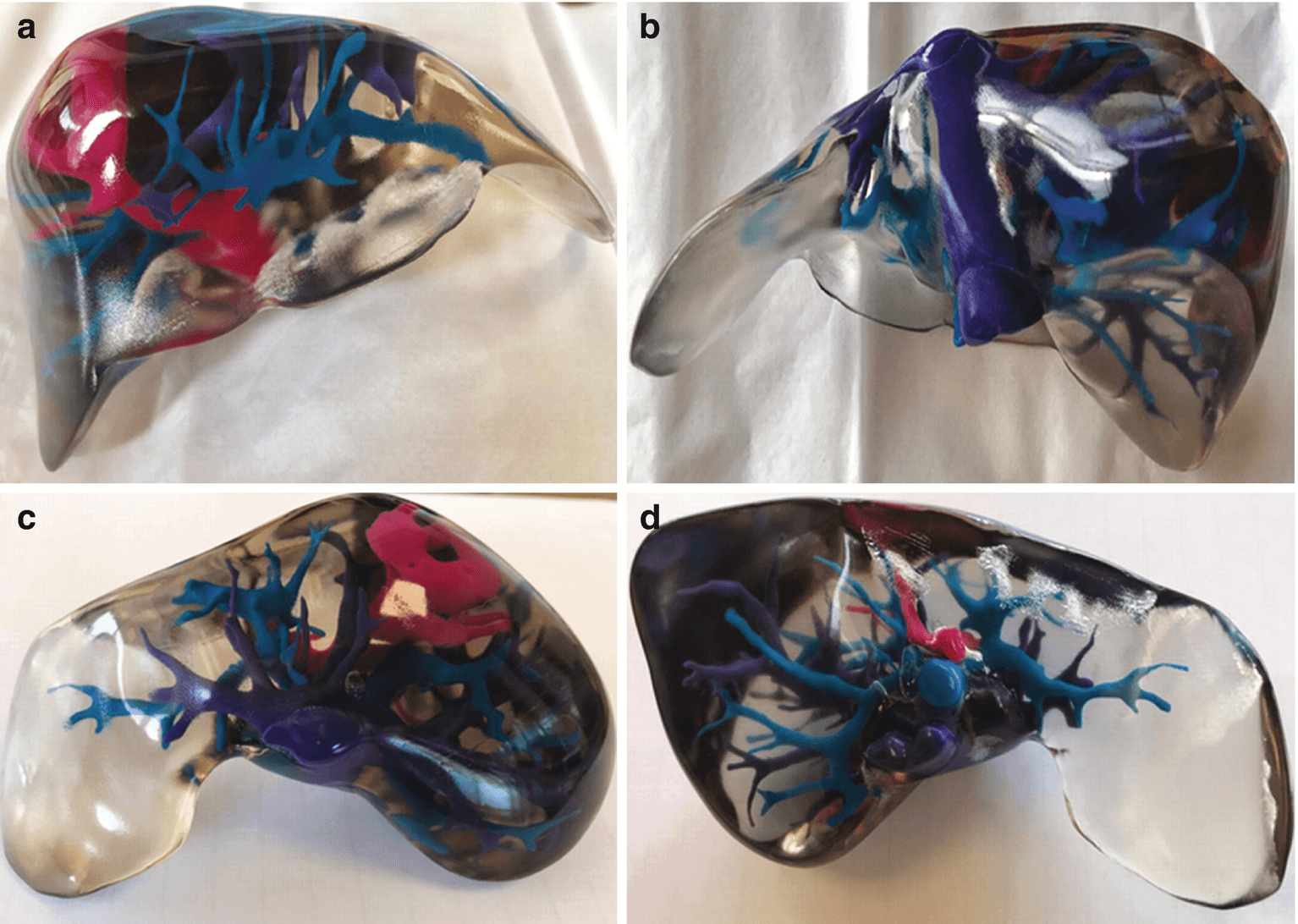
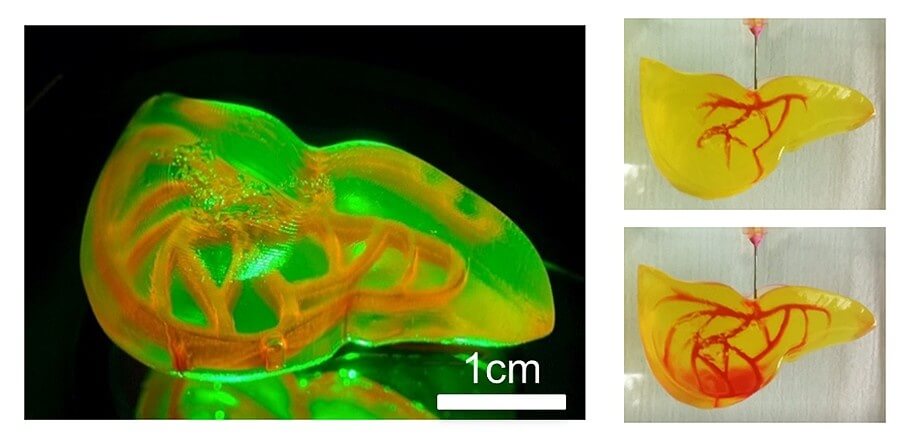
The findings can be found in the team’s paper titled “Fast Stereolithography Printing of Large‐Scale Biocompatible Hydrogel Models” and mark a significant leap for the printing of human organs, a notoriously slow and delicate process. Notably, the researcher’s new approach uses a combination of a 3D printing process called stereolithography that uses a laser beam to fabricate a structure layer by layer, and hydrogels that act as cell support while facilitating the formation of an ideal tissue. Aside from being fast and efficient, the method is perfect for printing cells with built-in blood vessel networks, and although it is currently limited to “centimeter-sized” models, it will no doubt become an essential steppingstone in the production of human-sized organs.
“Our method allows for the rapid printing of centimeter-sized hydrogel models,” explained Chi Zhou, the study’s lead co-author. “It significantly reduces part deformation and cellular injuries caused by the prolonged exposure to the environmental stresses you commonly see in conventional 3D printing.”
Large-size cell‐laden hydrogel models hold great promise for tissue repair and organ transplantation, but their fabrication using 3D bioprinting is defined by the slow printing rate that can affect the printed organ and the biological activity of the encapsulated cells. The fast hydrogel stereolithography printing (FLOAT) method is presented as allowing the creation of a centimeter‐sized, multiscale solid hydrogel model within minutes. Through precisely controlling the photopolymerization condition, low suction force‐driven, the high‐velocity flow of the hydrogel prepolymer is established that supports the continuous replenishment of the prepolymer solution below the curing part and the nonstop part growth.
Embedded vessel networks fabricated through multiscale printing allow media perfusion needed to maintain the high cellular viability and metabolic functions in the deep core of the large‐sized models. The endothelialization of this vessel network allows the institution of barrier functions. Together, these studies show a rapid 3D hydrogel printing method and represent a first step toward the fabrication of large‐sized engineered tissue models.
The healthcare industry is no stranger to 3D printing. It has helped tissue culture, facilitated the development of research on neurological diseases, with 3D printing medical advances gaining public scrutiny through the creation of 3D medical organs. The 3D printing of organs became a hot topic in 2015 when a three-year-old from Ireland become the first to have a life-saving adult kidney transplant courtesy of pioneering 3D printing used at Guy’s and St Thomas’ NHS Foundation Trust.
In the meantime, according to the United Network for Organ Sharing, just 39.000 transplants were performed in 2020 in the US, while more than 107.000 people are on the transplant waiting list as of February 2020. Out of these, approximately 17 die each day due to lack of organ compatibility or available donors. As such, the quick development of new tissues with 3D printing would ensure a steady supply of compatible organs for the patients without the long waiting time and uncertainty of whether the respective organ is a match.
Follow TechTheLead on Google News to get the news first.