With so much bad news about the Omicron variant and what the future might mean, this next announcement might be a ray of sunshine.
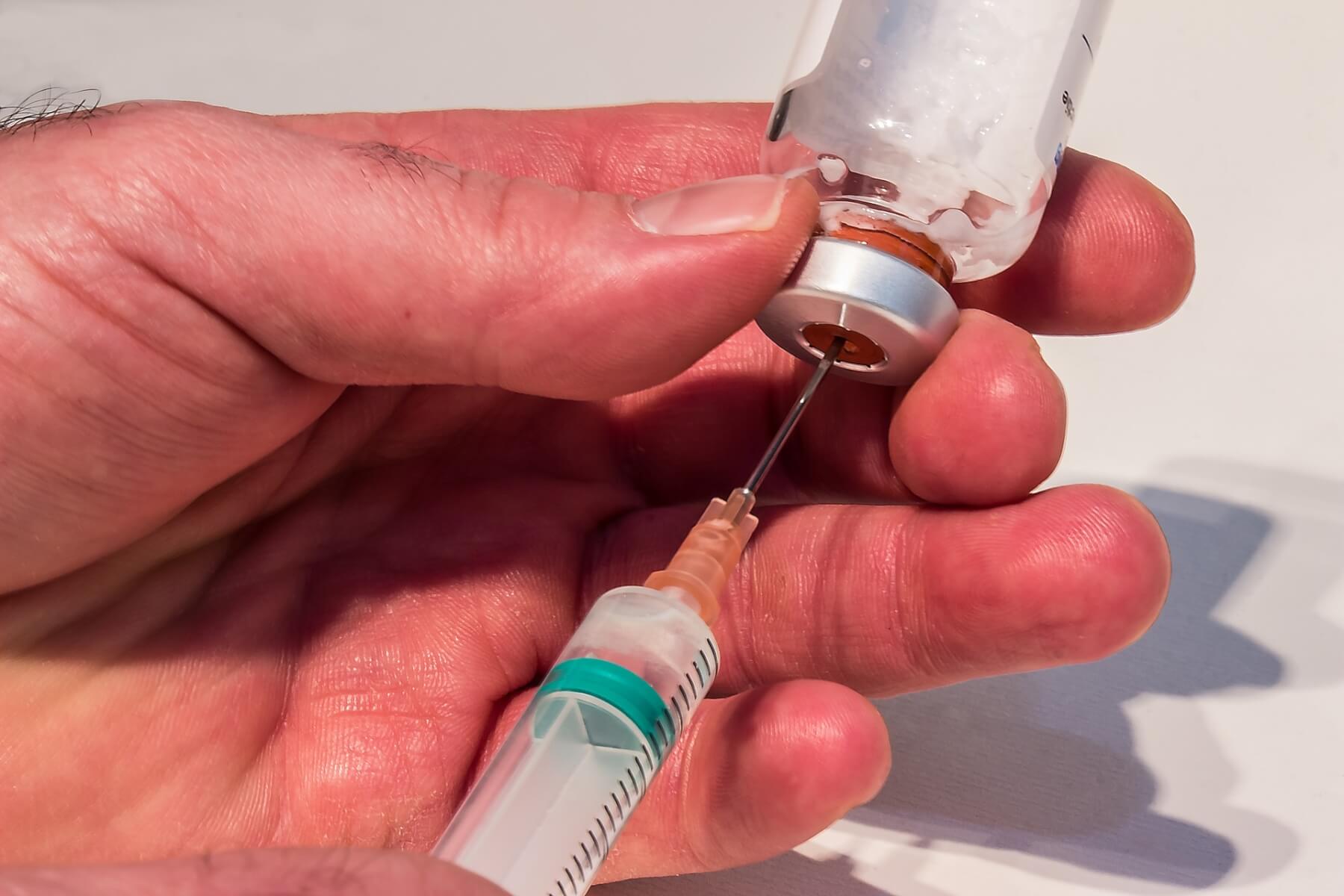
The FDA just approved the world’s first injectable medication that can reduce the risk of acquiring HIV.
The injectable medicine comes as an alternative to daily pills like Truvada and Descovy, which are 99% effective at preventing infection, and could revolutionize the way people are kept safe.
Unlike daily pills, Apretude requires just two shots, one month apart, then another injection every two months after in order to prevent infection.
Apretude, with the generic name “cabotegravir extended-release injectable suspension”, has the potential to be crucial in the fight against HIV spread, especially in helping high-risk individuals or people who can’t reliably take daily pills. The number of potential users of this new drug could be in the millions.
Pre-exposure prophylaxis (PrEP) medications that can prevent HIV are recommended for about 1.2 million people in the US and prescribed to up to a quarter of those people.
“This injection, given every two months, will be critical to addressing the HIV epidemic in the US, including helping high-risk individuals and certain groups where adherence to daily medication has been a major challenge or not a realistic option,” said Dr. Debra Birnkrant, director of the Division of Antivirals in the FDA’s Center for Drug Evaluation and Research.
Based on two clinical trials, randomized and double-blind, Apretude can reduce the risk of HIV infection more effectively than daily pills like Truvada.
The first trial included 4,600 cisgender men and transgender women engaging in intercourse with men, and the results showed a 69 percent lower risk of infection as compared to Truvada.
The second trial, done with the participation of 3,200 cisgender woman at risk of HIV infection, found that those poeple on Apretude had a 90 percent lower risk of infection as compared with the people on Truvada.
According to NBC News, Apretude has a list price of US$3,700 per dose (or $22,200 per year for six doses) and will start shipping next year. As for insurance, that now covers Truvada and Descovy, another daily pill, but no provisions have been made for the HIV prevention shot.
Surgeons Successfully Transplanted A Pig Kidney Into A Human For the First Time
Follow TechTheLead on Google News to get the news first.