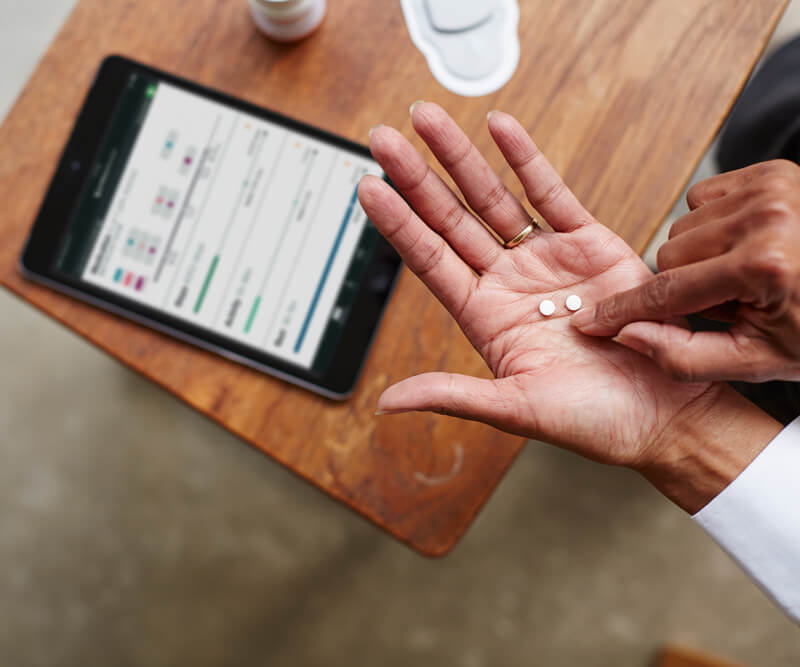
The Food and Drug Administration have just approved the first digital pill, a drug that contains an ingestible sensor that can track precious information about a patient #sciencemagic
Abilify MyCite is a small pill with a tiny sensor that communicates with an exterior patch. The sensor is made from silicon, copper and magnesium and once it detects stomach acid, it sends an electrical signal in the body. The sensor is eliminated in a natural way, while the patch is smart enough to get the signal a couple of minutes after the pill is taken by the patient.
The patch is responsible with transmitting the data collected to a smartphone app via Bluetooth. Abilify MyCite takes into account activity levels, sleeping patterns, steps taken, activity, and heart rate. All of this can be accessed by the patient’s doctor and close family or the people the patient chooses fit. Of course, Abilify does take care of illness, as a drug would. It treats schizophrenia, bipolar disorder, and is can be taken for depression.
The drug is the product of a partnership between pharmaceutical company Otsuka and Proteus Digital Health, the ones that make the sensor. It’s the first of more drugs the FDA plans to review, which is also the reason why they’re looking to hire staff with a deeper understanding of software development as well as medicine.
The Abilify pill price is still a mystery and production is set to ramp up only if the manufacturers can find insurers to cover the pills.
Follow TechTheLead on Google News to get the news first.